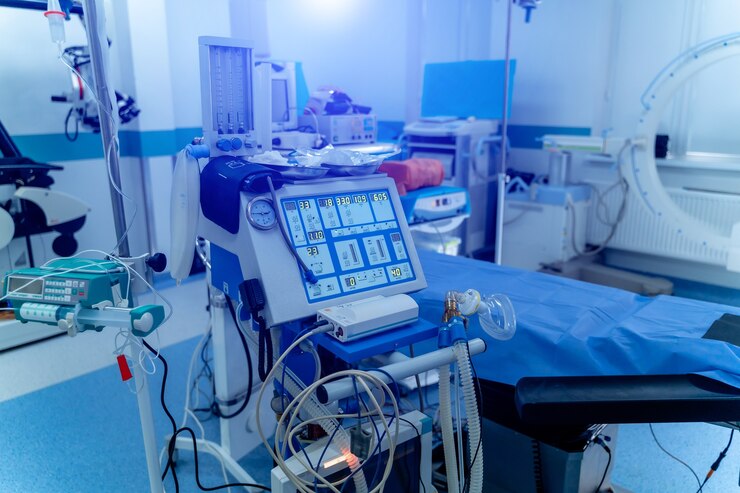
Contract Manufacturing
Contract Manufacturing of Medical Equipment: Driving Innovation and Efficiency in Healthcare
In today’s fast-evolving healthcare industry, precision, quality, and innovation are essential. Medical device companies, from startups to established manufacturers, increasingly rely on contract manufacturing to meet the demands of the global market. Contract manufacturing of medical equipment involves outsourcing the production, assembly, testing, and even packaging of medical devices to a specialized third-party manufacturer. This strategic partnership allows medical device companies to focus on core competencies such as R&D, marketing, and regulatory compliance, while benefiting from the operational strengths of experienced manufacturers.


What Is Contract Manufacturing in Medical Equipment?
Contract manufacturing in the medical field refers to a business model where a medical device company partners with an external manufacturer to produce equipment under their brand. This may include complete device assembly, sub-assembly production, or manufacturing of individual components.
The scope of services often extends to prototyping, engineering support, sterilization, labeling, and even logistics. Such manufacturers must comply with stringent standards, including ISO 13485 certification, FDA regulations (21 CFR Part 820), and other global regulatory frameworks depending on the markets being served. The ability to meet these requirements makes contract manufacturing an essential element in the medical supply chain.
Key Benefits of Medical Equipment Contract Manufacturing
Cost Efficiency
Setting up a manufacturing facility for medical devices requires significant capital investment, not just in infrastructure but also in equipment, regulatory compliance, and skilled labor. Contract manufacturers already possess these resources. By leveraging their infrastructure, medical companies can reduce overhead and operational costs.
Access to Expertise and Technology
Contract manufacturers specialize in the production of complex medical devices and components. Their teams include engineers, technicians, quality assurance specialists, and regulatory experts who ensure high precision and compliance. These manufacturers also stay up to date with the latest technological advancements in automation, robotics, and materials science, helping clients innovate without the upfront costs.
Speed to Market
the competitive healthcare space, speed matters. Outsourcing production can significantlyreduce time-to-market by overlapping design, prototyping, and manufacturing processes. Contract manufacturers often offer flexible production lines and rapid prototyping capabilities that accelerate development cycles.
Scalability
From initial pilot runs to full-scale commercial production, contract manufacturing partners offer scalable solutions that grow with your business. Whether a company needs small batches for clinical trials or large-scale production for global distribution, contract manufacturers can adapt
to changing demands.
Risk Reduction
Manufacturing medical equipment involves numerous risks, from regulatory compliance issues to production inefficiencies. Contract manufacturers have the experience and systems in place to mitigate these risks, ensuring quality and consistency across the product lifecycle.
Commonly Outsourced Medical Devices and Components
Contract manufacturers handle a wide variety of medical equipment, including:
● Diagnostic imaging devices
● Surgical instruments
● Catheters and tubing systems
● Orthopedic implants
● Patient monitoring systems
● Wearable medical devices
● Laboratory equipment
● Disposable medical supplies
Components such as electronic circuitry, molded plastic parts, metal components, and sterilized packaging materials are also frequently produced by contract manufacturers.
Choosing the Right Contract Manufacturing Partner
Selecting the right partner is crucial. Here are a few factors to consider:
● Certifications and Compliance: Ensure the manufacturer meets necessary standards like ISO 13485 and FDA requirements.
● Experience: Look for a proven track record in your device category or market.
● Technological Capabilities: Assess their ability to support complex designs and scale production.
● Communication and Transparency: A strong partnership relies on clear communication and trust.
● Location: Consider proximity to your R&D team or distribution network, especially for logistics-sensitive products.
Contract manufacturing has become an integral part of the medical device industry, empowering companies to bring innovative products to market more quickly, cost-effectively, and with higher quality. With the right partner, medical equipment companies can enhance operational efficiency, reduce risk, and focus on what they do best: improving patient care through cutting-edge medical solutions.